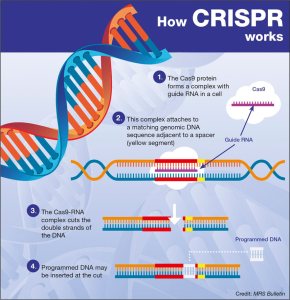
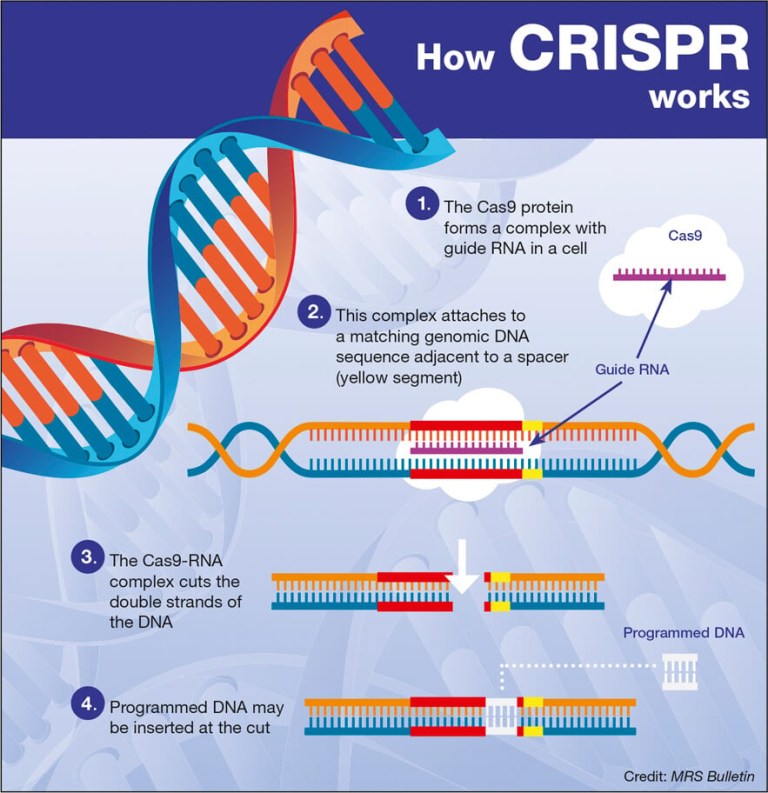
Alzheimer’s research is at the forefront of one of the most pressing health challenges of our time, aiming to unravel the complexities of this devastating neurodegenerative disease. Scientists like Beth Stevens at Boston Children’s Hospital are pioneering studies that scrutinize the role of microglial cells—the brain’s immune system—in maintaining neuronal health and function. These tiny cells are crucial for clearing out damaged cells and ensuring synapses are properly maintained. However, Stevens’ groundbreaking findings highlight how improper pruning by microglia can exacerbate Alzheimer’s and similar disorders, opening the door to potential new treatments and early biomarkers. As the number of Alzheimer’s cases in the U.S. continues to surge, with projections of reaching 7 million by 2050, advancements in this research are critical in the fight against cognitive decline and improving patient care.
The exploration of Alzheimer’s disease encompasses a broader spectrum of understanding concerning cognitive degeneration and the intricacies of the brain’s defensive mechanisms. Scientists are delving deeply into how the brain’s immune response, particularly through microglial activation, contributes to the development of neurological disorders. This research, highlighted by key figures like Beth Stevens, pulls from various lines of inquiry, shedding light on critical pathways that link neuroimmune interactions with disease progression. As these insights into the brain’s protective system emerge, the possibility of innovative therapies for neurodegenerative diseases grows exponentially, lending hope to millions affected by conditions like Alzheimer’s. By understanding the role of these immune cells, researchers aim to revolutionize the landscape of treatment and detection in an aging population.
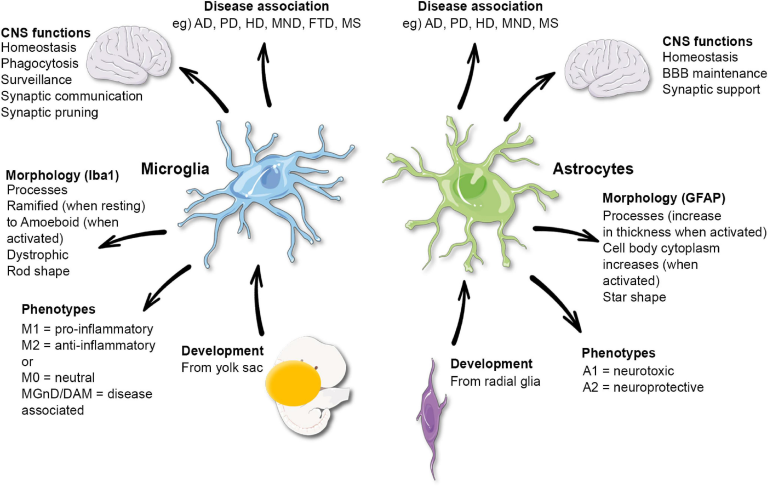
Understanding Microglial Cells and Their Role in Alzheimer’s Research
Microglial cells play a critical role in maintaining brain health, acting as the first line of defense within the brain’s immune system. These cells are responsible for monitoring the cellular environment and respond to injury or disease by removing damaged neurons and debris. In the context of Alzheimer’s disease, recent findings from Beth Stevens’ lab have highlighted how dysregulation in microglial activity can lead to excessive pruning of synapses, disrupting communication pathways between neurons. This understanding is vital as it lays the groundwork for potential therapies aimed at restoring normal microglial function, thereby mitigating disease progression.
Stevens’ innovative research has illustrated that microglial cells are not only involved in response to injury but are actively engaged in the complex process of synaptic pruning during normal brain development. By identifying the mechanisms by which these immune cells can contribute to neurodegenerative diseases, researchers can better understand not just Alzheimer’s but also other disorders such as Huntington’s disease. This shift in perspective opens up new avenues for targeted treatments and early diagnostics, crucial in the fight against Alzheimer’s and related conditions.
Beth Stevens: Pioneering Breakthroughs at Boston Children’s Hospital
Beth Stevens has positioned herself at the forefront of Alzheimer’s research, leveraging her role at Boston Children’s Hospital and the Broad Institute to propel critical discoveries about the brain’s immune system. Her work is characterized by a merger of rigorous scientific inquiry and innovative thinking, which has led to significant understanding of microglial function in neurodegenerative diseases. As she articulates, her journey has been driven by a fundamental curiosity about how the brain’s immune cells interact within the neural framework, ultimately impacting synaptic health and plasticity.
The Stevens Lab exemplifies how curiosity-driven science can lead to transformative insights in medicine. With a robust funding structure supported by federal agencies such as the National Institutes of Health, the lab has focused on unraveling the complexities of microglia’s roles in diseases like Alzheimer’s. Through extensive research, they have not only shed light on pathogenic mechanisms but also begun to pioneer the development of novel therapeutic strategies aimed at correcting microglial dysfunction, further emphasizing the critical intersection of basic and applied science.
The Future of Alzheimer’s Disease Treatment: Innovations Through Microglial Research
As Alzheimer’s disease continues to pose a significant challenge to public health, the need for innovative treatment approaches has never been more urgent. Stevens’ recent studies emphasize the potential for harnessing microglial cells in developing new treatments. By understanding how these immune cells misfunction in Alzheimer’s, researchers aim to create targeted therapies that can restore their normal activity, improving the overall neural environment and potentially slowing disease progression. This research could also pave the way for the identification of early biomarkers, enabling timely intervention.
Innovations stemming from the Stevens Lab’s research may not only disrupt the traditional understanding of Alzheimer’s but could also transform therapeutic strategies for various neurodegenerative diseases. By focusing on microglial cell dynamics and their influence on synaptic health, scientists can address the underlying causes of these disorders instead of merely managing symptoms. This paradigm shift underscores the importance of ongoing research in understanding the brain’s immune system, which may lead to more effective treatments for the millions affected by Alzheimer’s and similar diseases.
The Role of Curiosity in Scientific Discovery: Lessons from Beth Stevens
The intricate relationship between scientific curiosity and genuine breakthroughs is exemplified by the work of Beth Stevens in Alzheimer’s research. Her phrase, ‘I was just following the science,’ encapsulates the spirit of inquiry that drives many in the field of neuroscience. It highlights how significant discoveries often come from asking seemingly simple questions and exploring the unexpected connections between immune functions and neurodegenerative disorders. This fundamental curiosity not only stimulates innovative research but can also lead to impactful health solutions.
Stevens’ approach underscores the importance of federal funding and institutional support in fostering environments where curiosity can thrive. Her recognition as a MacArthur ‘genius’ emphasizes that breakthroughs in science are not just the result of structured plans but emerge from a willingness to explore the unknown. By advocating for continued support for basic research, Stevens champions the notion that today’s inquiries will lay the foundation for tomorrow’s therapeutic advancements, especially concerning Alzheimer’s and its associated challenges.
Exploring the Synaptic Landscape: How Microglia Influence Brain Connectivity
The synaptic landscape of the brain is complex and ever-changing, influenced significantly by microglial activity. These cells are unique in that they constantly adapt to the brain’s needs, performing essential tasks in shaping neural connectivity through processes like synaptic pruning. In Alzheimer’s disease, excessive pruning can disrupt these connections, leading to cognitive decline. Research conducted by Beth Stevens highlights the importance of maintaining a healthy balance of synaptic strength and connectivity, as aberrations in this balance can forecast neurodegenerative outcomes.
Understanding how microglia interact with neurons and affect synaptic health not only provides insights into the mechanisms of Alzheimer’s but also informs potential treatment methodologies. By targeting the pathways through which microglia execute synaptic pruning, there lies a potential to develop interventions that could correct or normalize synaptic loss. This line of inquiry is essential for researchers aiming to combat the multifactorial nature of Alzheimer’s disease, as it integrates knowledge of immune function with neurobiology.
Microglia and Neurodegeneration: Insights for Future Research
As research continues to unravel the complexities of neurodegenerative diseases, the role of microglial cells stands out as a critical area of study. The aberrant activity of these immune cells has been implicated in conditions like Alzheimer’s and Huntington’s disease, emphasizing the need for deeper understanding and investigation. Beth Stevens’ findings bolster the hypothesis that microglial dysfunction is not merely a byproduct of neuronal damage but rather a central component of disease pathology. This connection underlines the potential for targeting microglia in therapeutic development.
Future research into microglial behaviors could transform how we approach the treatment of neurodegenerative diseases. By elucidating the specific mechanisms through which microglia contribute to neuron health and disease progression, scientists can develop targeted therapies aimed at restoring normal functions. Such innovations could pave the way for preventative measures, potentially altering the trajectory of diseases like Alzheimer’s for millions, hence highlighting why ongoing investigation in this field is paramount.
Advancements in Alzheimer’s Biomarkers: The Role of Microglia
The quest for effective Alzheimer’s biomarkers hinges upon understanding the underlying mechanisms of the disease. Microglial activity provides a promising avenue for the identification of such biomarkers. With ongoing research led by pioneers like Beth Stevens, scientists are exploring how changes in microglial function might correlate with the disease’s onset and progression. Identifying such markers can facilitate earlier diagnosis and intervention, significantly altering patient outcomes.
The potential development of biomarkers based on microglial behavior can revolutionize our approach to Alzheimer’s management. By correlating specific microglial actions with clinical manifestations of Alzheimer’s, researchers may not only enhance diagnostic precision but also tailor therapeutic approaches based on individual immunological profiles. This level of personalized medicine could be transformative in the field of neurodegenerative disease, underscoring the importance of interdisciplinary collaboration in research.
Federal Support in Alzheimer’s Research: A Conversation with Beth Stevens
Federal support plays a critical role in advancing Alzheimer’s research, as highlighted by the trajectory of Beth Stevens’ work. With backing from the National Institutes of Health, her lab has been able to delve into the fascinating relationship between microglial cells and neurodegenerative diseases. This funding not only affirms the importance of basic science in understanding complex biology but also ensures that innovative ideas can transition into practical applications that could mitigate the impact of Alzheimer’s disease in the aging population.
Support from federal agencies is essential for fostering groundbreaking discoveries in the realms of neuroscience and immunology. As Stevens emphasizes, without this foundation of funding, the journey from hypothesis to treatment could be severely hindered. Such sustained investment into Alzheimer’s research ensures a steady pipeline of innovations that hold the promise of new therapies, demonstrating the indispensable role of public funding in shaping the future of healthcare.
Frequently Asked Questions
How do microglial cells relate to Alzheimer’s research?
Microglial cells play a crucial role in Alzheimer’s research as they act as the brain’s immune system. They monitor and maintain brain health by removing dead cells and debris. Recent studies indicate that dysfunctional microglial activity, such as improper synapse pruning, may contribute to neurodegenerative diseases like Alzheimer’s, highlighting their importance in ongoing research efforts to develop new treatments.
What breakthroughs has Beth Stevens made in Alzheimer’s research?
Beth Stevens has made significant contributions to Alzheimer’s research by demonstrating how microglial cells improperly prune synapses, which can lead to Alzheimer’s disease. Her work at Boston Children’s Hospital and the Broad Institute has helped illuminate the role of these immune cells in neurodegenerative diseases, paving the way for potential new therapies and biomarkers to detect Alzheimer’s earlier in patients.
What is the significance of the brain immune system in Alzheimer’s research?
The brain immune system, primarily composed of microglial cells, is significant in Alzheimer’s research because it helps regulate brain health. By studying how these immune cells respond during neurodegenerative diseases, researchers like Beth Stevens have uncovered mechanisms that lead to Alzheimer’s, which could inform future treatment strategies aimed at modulating immune responses to protect against neuronal damage.
How are neurodegenerative diseases studied in relation to microglia?
Neurodegenerative diseases like Alzheimer’s are studied in relation to microglia by investigating how these cells react to neuronal changes. Researchers analyze the pruning activities of microglia and their effects on synaptic structures, revealing how miscommunication or malfunction of these immune cells can exacerbate conditions such as Alzheimer’s, thus providing insights into potential therapeutic interventions.
What role does Boston Children’s Hospital play in Alzheimer’s research?
Boston Children’s Hospital, under the leadership of researchers like Beth Stevens, plays a vital role in Alzheimer’s research by focusing on the functions of microglial cells and their implications in neurodegenerative disease. Their collaborative work with institutions like the Broad Institute facilitates innovative research aimed at understanding and ultimately treating Alzheimer’s disease and related disorders.
Why are new biomarkers important in Alzheimer’s research?
New biomarkers are critical in Alzheimer’s research as they enable earlier detection and intervention for the disease. By identifying specific biological indicators associated with neurodegenerative processes, researchers can monitor disease progression and response to treatments more effectively, which is essential for improving patient outcomes and managing future healthcare costs related to Alzheimer’s.
| Key Points |
|---|
| Beth Stevens is a neuroscientist focused on Alzheimer’s research, particularly on microglial cells’ role in brain health. |
| Microglia act as the brain’s immune system, clearing dead cells and maintaining synaptic health, but their malfunction may lead to neurodegenerative diseases. |
| The Stevens Lab’s findings link improper synaptic pruning to Alzheimer’s and other disorders, providing a foundation for new therapies and early detection biomarkers. |
| An estimated 7 million Americans are affected by Alzheimer’s, with projections suggesting this number could double by 2050, drastically increasing healthcare costs. |
| Stevens emphasizes the importance of basic and curiosity-driven research, which can lead to significant medical advancements and improved understanding of diseases. |
Summary
Alzheimer’s research is at the forefront of neuroscience, with scientists like Beth Stevens making significant breakthroughs. By investigating the role of microglial cells in the brain, Stevens has uncovered vital connections between their function and neurodegenerative diseases, including Alzheimer’s. Her work not only enhances our understanding of disease mechanisms but also paves the way for developing innovative treatments and early detection strategies to combat this growing public health challenge.