TIM-3 therapy for Alzheimer’s is emerging as a groundbreaking approach in the quest for effective Alzheimer’s treatment. By targeting a specific immune checkpoint molecule, TIM-3 therapy aims to unleash the full potential of microglia, the brain’s immune cells, allowing them to effectively attack amyloid plaques that contribute to cognitive decline. Recent studies have shown promising results in mice, where inhibiting TIM-3 expression led to an improved microglia function and cognitive recovery, highlighting the potential of this therapy to alter the course of Alzheimer’s disease. With the alarming rise in Alzheimer’s cases globally, innovative strategies like TIM-3 therapy could reshape how we approach this challenging condition. As researchers continue to explore the immune system and Alzheimer’s relationship, advancements in TIM-3 therapy may offer new hope to millions affected by this devastating disease.
The innovative strategy of targeting TIM-3 in Alzheimer’s disease represents a vital step towards novel interventions for memory impairment linked to aging. This therapy focuses on immune checkpoint molecules, which, when inhibited, can enhance the functionality of microglial cells, essential players in brain health. By altering the dynamics of these immune responders, scientists are exploring a pathway that could lead to significant cognitive recovery for those suffering from Alzheimer’s. Considering that a large percentage of Alzheimer’s cases are late-onset, the implications of TIM-3 therapy could be monumental for patient outcomes. As researchers delve deeper into the complex relationship between the immune system and Alzheimer’s, we may stand on the brink of transformative advancements in treatment options.
Understanding the Role of TIM-3 in Alzheimer’s Disease
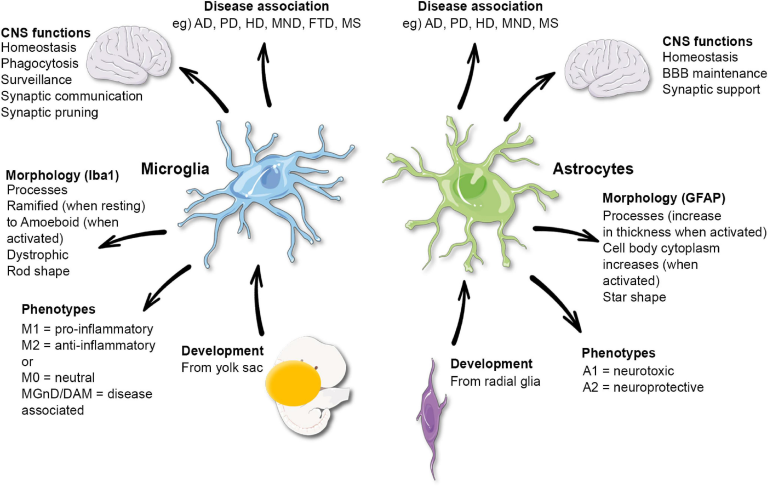
TIM-3, or T cell immunoglobulin and mucin domain-3, is a checkpoint molecule that has gained considerable attention for its inhibitory effects on microglial cells in the context of Alzheimer’s disease (AD). Research indicates that in patients with late-onset Alzheimer’s, TIM-3 is overexpressed on microglia, the brain’s resident immune cells. This overexpression hinders microglia’s ability to clear amyloid-beta plaques, which accumulate and contribute to cognitive decline. In studies, the deletion of TIM-3 in mouse models led to enhanced clearance of these plaques and subsequent improvements in memory performance, highlighting its potential as a therapeutic target in Alzheimer’s treatment.
The biological mechanisms underlying TIM-3’s role in AD involve its function as a checkpoint inhibitor, which in normal immune responses, serves to prevent overactivation and tissue damage. In the context of Alzheimer’s, however, the inhibitory pathways activated by TIM-3 may contribute to the failure of microglia to perform their crucial role in synaptic maintenance and debris clearance. Understanding how TIM-3 modulation can reset microglial activity opens new avenues for Alzheimer’s treatment, aiming not just to alleviate symptoms but to address the root causes associated with plaque accumulation.
The Implications of Checkpoint Molecules in Alzheimer’s Treatment
Checkpoint molecules, including TIM-3, have traditionally been recognized in the context of cancer therapy, where they play a role in regulating immune responses against tumors. With recent findings, however, there is a growing recognition of their importance in neurodegenerative diseases like Alzheimer’s. As researchers explore anti-TIM-3 therapies, they hope to leverage the knowledge gained from cancer immunotherapy to develop innovative approaches for Alzheimer’s treatment. This could eventually lead to more effective interventions that enhance cognitive recovery by facilitating the clearance of neurotoxic plaques.
The duality of checkpoint molecules serves as both a shield for the body and a burden in neurodegenerative contexts. By activating certain checkpoint pathways, cancer cells evade immune detection; similarly, in Alzheimer’s disease, an overactive checkpoint response may be preventing beneficial immune activity. The challenge lies in designing therapeutics that can selectively modulate these checkpoints, thereby restoring normal microglial function without compromising the overall immune balance. Such an approach could mark a fundamental shift in how Alzheimer’s is treated, perhaps shifting focus from merely symptom management to fostering genuine cognitive recovery.
Exploring Microglia Function in Alzheimer’s Disease
Microglia play a pivotal role in maintaining brain health, acting as the primary immune defenders of the central nervous system. In the context of Alzheimer’s disease, their functions are compromised due to the overexpression of inhibitory molecules like TIM-3. This inhibition prevents microglia from effectively removing amyloid plaques and debris, contributing to the ongoing cycle of neurodegeneration. Studies indicate that enhancing microglial function by targeting TIM-3 may activate these cells to resume their protective duties, potentially slowing disease progression.
Additionally, understanding microglial function in Alzheimer’s opens up opportunities for novel therapeutic strategies. By focusing on restoring microglial activity through TIM-3 inhibition, researchers aim to not only reduce amyloid plaque burden but also promote a favorable environment for neuronal health and cognitive function. As the field progresses, the identification of pathways to rejuvenate microglial activity will be crucial in developing targeted treatments that address both the symptoms and the underlying pathology of Alzheimer’s disease.
Cognitive Recovery: The Potential of TIM-3 Modulation
Cognitive recovery in Alzheimer’s patients is a foremost goal in therapeutic development. Recent research into TIM-3 modulation demonstrates promise in enhancing cognitive capabilities through targeted immunotherapy. Mice genetically modified to lack TIM-3 show significant improvement in memory tasks, suggesting that inhibiting this checkpoint molecule could facilitate better synaptic health and enhance cognitive functions. Such findings provide a foundation for exploring TIM-3 therapy as a viable approach to restore cognitive functions in humans.
The potential for restoring cognitive recovery via TIM-3 modulation offers hope to millions affected by Alzheimer’s disease. By enhancing microglial clearance of plaques and rejuvenating synaptic health, TIM-3 therapy could lead to measurable improvements in cognitive performance. The translation of these findings from animal models to human applications will be critical, and ongoing trials will help ascertain the safety and efficacy of TIM-3-targeted treatments for improving cognitive function in Alzheimer’s patients.
The Immune System and Alzheimer’s Disease: A New Perspective
The intricate connection between the immune system and Alzheimer’s disease is becoming increasingly evident. TIM-3 functions not only as a marker for immune regulation but also as a crucial determinant of microglial activity in the brain. By inhibiting the signaling pathways of TIM-3, researchers are seeking to redefine the immune response in Alzheimer’s, enabling a more aggressive approach to tackle amyloid plaque buildup. This perspective shift could lead to potential breakthroughs in how we understand and treat Alzheimer’s.
Reassessing the role of the immune system in Alzheimer’s could also pave the way for combination therapies that integrate immunotherapies with existing treatment modalities. By targeting checkpoint molecules like TIM-3, it may be possible to enhance the efficacy of other treatments, thus providing a multifaceted approach to combatting Alzheimer’s disease. As the research evolves, understanding the immune system’s influence on neurodegeneration will be crucial in developing comprehensive treatment strategies.
Cancer Therapies and Their Relevance to Alzheimer’s Research
The strategies used in cancer immunotherapy are informing new approaches in the treatment of Alzheimer’s disease, especially regarding checkpoint inhibitors like TIM-3. While such therapies have primarily been applied to cancer, their principles can be adapted to enhance immune responses against amyloid plaques in Alzheimer’s patients. The intersection of these two fields opens exciting possibilities for developing innovative therapeutic strategies that leverage the immune system’s potential to combat both cancer and neurodegenerative diseases.
Innovative approaches inspired by cancer therapy underscore the importance of a robust immune response in neurodegenerative conditions. By strategically targeting TIM-3 with specific antibodies or small molecules, it may be possible not just to clear amyloid plaques, but fundamentally alter the course of Alzheimer’s disease. Ongoing research may eventually lead to dual-purpose therapies that address both tumor cells and neurodegenerative plaques, representing a significant advance in our understanding of immune modulation in complex diseases.
Future Research Directions in Alzheimer’s Immunotherapy
The future of Alzheimer’s research lies in the innovative exploration of immunotherapeutic strategies that target molecules like TIM-3. As scientists develop anti-TIM-3 antibodies and investigate their capacity to promote microglial activity, the potential for groundbreaking advances in Alzheimer’s treatment becomes evident. This line of research may lead to new therapies that not only slow disease progression but also improve cognitive function in affected individuals.
Future studies will be essential to refine these therapies for optimal efficacy and safety in human patients. By focusing on the role of checkpoint molecules such as TIM-3 in the neuroinflammatory response associated with Alzheimer’s, researchers are poised to unravel new pathways for intervention. Collaborations between institutions and advancements in genetic engineering will aid in tailoring therapies that specifically target Alzheimer’s pathology, ushering in a new era of hope for patients and their families.
The Role of Genetic Factors in Alzheimer’s Disease and TIM-3
Genetic predispositions play a significant role in the development of Alzheimer’s disease, with TIM-3 emerging as a notable candidate gene linked to late-onset AD. The polymorphisms within the TIM-3 gene represent a critical risk factor for Alzheimer’s, influencing the expression and function of this checkpoint molecule in microglia. Understanding the genetic landscape surrounding TIM-3’s role in Alzheimer’s could lead to personalized approaches in treatment, tailoring therapies based on individual genetic profiles.
Research on genetic factors in Alzheimer’s also underscores the importance of early intervention. By identifying individuals at risk due to genetic markers like those associated with TIM-3, proactive measures can be taken to mitigate the onset of symptoms. Exploring genetic influences expands our understanding of Alzheimer’s and could significantly influence the development of targeted therapies aimed at specific populations, ultimately improving treatment outcomes.
The Challenge of Drug Delivery in TIM-3 Therapy
One of the significant hurdles in developing TIM-3 therapy for Alzheimer’s disease lies in the effective delivery of therapeutic agents to the brain. Current treatments often face barriers due to the blood-brain barrier’s selective permeability, limiting the efficacy of administered antibodies. Innovative drug delivery systems that facilitate the precise targeting of TIM-3 in brain tissue will be crucial. Research focusing on nanoparticles and other delivery mechanisms promises to enhance treatment penetration and specificity, ensuring that anti-TIM-3 therapies reach their intended targets.
Addressing these drug delivery challenges is paramount for the success of TIM-3 therapy in clinical settings. By exploring methods to enhance the bioavailability of drugs within the central nervous system, researchers aim to optimize therapeutic outcomes while minimizing side effects. As the field advances, overcoming these delivery obstacles could significantly improve the practicality of TIM-3 inhibitors in Alzheimer’s treatment, contributing to a new standard of care for this devastating disease.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s involves the use of an anti-TIM-3 antibody or a small molecule that inhibits the TIM-3 checkpoint molecule, which has been found to prevent microglia from attacking and clearing amyloid plaques in the brain. By blocking TIM-3, this therapy may enable microglia, the brain’s immune cells, to engage more effectively with plaques, thereby potentially improving memory function.
How does TIM-3 relate to Alzheimer’s treatment and cognitive recovery?
TIM-3 is a checkpoint molecule that inhibits microglial function. In Alzheimer’s treatment, targeting TIM-3 can enhance cognitive recovery by allowing microglia to clear harmful amyloid plaques from the brain, thus facilitating better memory retention and cognitive function.
What role do checkpoint molecules like TIM-3 play in the immune system and Alzheimer’s?
Checkpoint molecules like TIM-3 are crucial in regulating the immune response by preventing immune cells from becoming overactive. In Alzheimer’s, increased expression of TIM-3 on microglia prevents these cells from effectively clearing amyloid plaques, contributing to disease progression and cognitive decline.
What are the benefits of inhibiting TIM-3 for Alzheimer’s patients?
Inhibiting TIM-3 may lead to reduced plaque accumulation in the brain and improve the function of microglia, ultimately enhancing cognitive recovery and memory in Alzheimer’s patients by allowing these immune cells to effectively clear amyloid plaques.
Is TIM-3 therapy for Alzheimer’s effective in preclinical studies?
Yes, in preclinical studies involving lab mice, deleting the TIM-3 gene significantly improved plaque clearance and cognitive behavior, suggesting that TIM-3 therapy could be an effective strategy in treating Alzheimer’s disease.
What is the potential future of TIM-3 therapy in human Alzheimer’s treatment?
The future of TIM-3 therapy in Alzheimer’s treatment looks promising, as researchers are currently testing human anti-TIM-3 antibodies in mouse models that closely mimic human Alzheimer’s. This could lead to potential new therapies for alleviating symptoms and slowing disease progression.
How long did research on TIM-3 therapy for Alzheimer’s take and who is involved?
The research on TIM-3 therapy for Alzheimer’s took five years and was conducted by a collaborative team led by Vijay Kuchroo and Oleg Butovsky, focusing on understanding the role of TIM-3 in microglial function and Alzheimer’s pathology.
What are the implications of TIM-3 expression levels in Alzheimer’s disease?
In Alzheimer’s disease, elevated levels of TIM-3 on microglia have been linked to reduced plaque clearance and impaired cognitive function. Understanding these implications aids in developing targeted therapies that could restore microglial function and improve cognitive health.
| Key Aspect | Details |
|---|---|
| Research Focus | Exploring TIM-3 therapy for Alzheimer’s disease. |
| Key Findings | Deleting TIM-3 improves microglial clearance of amyloid plaques, enhancing memory in mice. |
| What is TIM-3? | A checkpoint molecule that inhibits immune response in the brain, contributing to plaque accumulation in Alzheimer’s. |
| Impact of TIM-3 Therapy | Potentially repurposes existing anti-TIM-3 treatments to promote plaque clearance and cognitive recovery. |
| Study Outcome | Mice with TIM-3 deleted showed reduced plaques and improved behavior, indicating recovery in cognitive functions. |
Summary
TIM-3 therapy for Alzheimer’s is a promising avenue that leverages immunotherapy principles to combat the disease. Recent studies indicate that by targeting the TIM-3 checkpoint molecule, researchers have observed significant improvements in microglial function, facilitating the removal of damaging amyloid plaques from the brain. This research not only underscores the potential for cognitive recovery in models of late-onset Alzheimer’s but also opens doors for innovative therapeutic strategies that may enhance the quality of life for those affected by this condition.